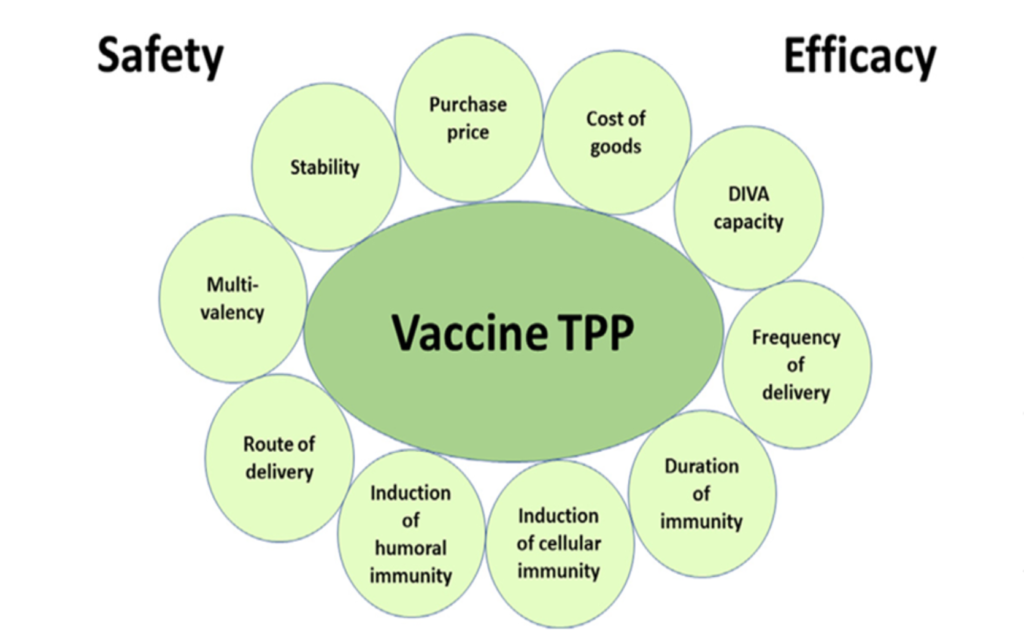
The first consideration for any vaccine development program should be the generation of a target product profile or TPP. This will outline the desired characteristics of the vaccine product that is under development. It generally identifies the “optimal target” and a “minimal target” and should include status updates of completed or planned studies that support the various new vaccine product targets.
Doing this enables the estimated targets to be refined as the project progresses, although typically the targets do not change unless the intended use of the product or required outcome changes or in response to regulatory guidance.
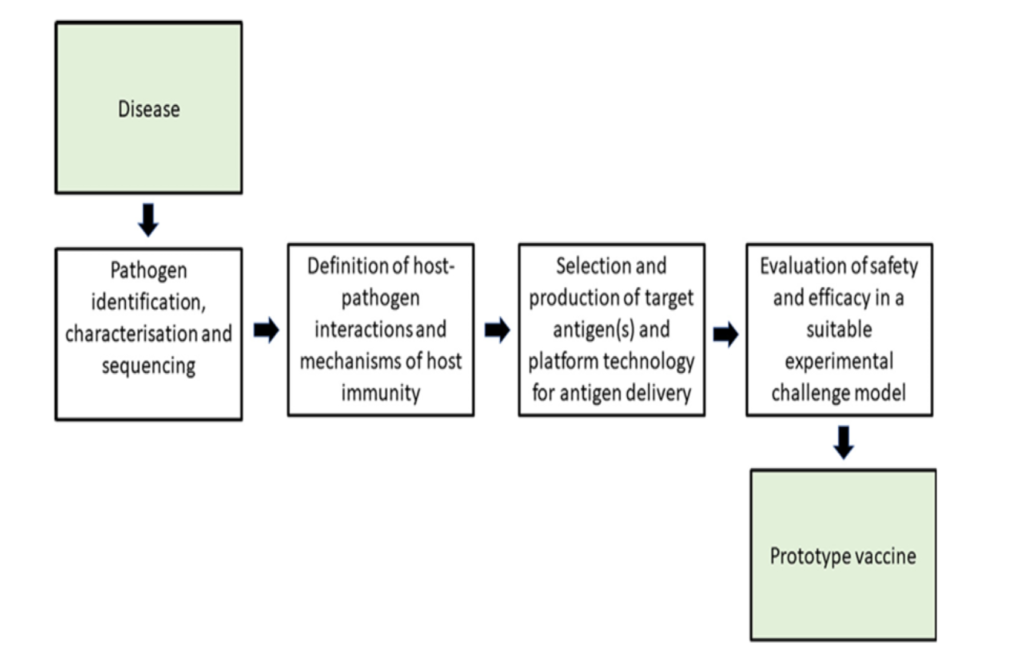
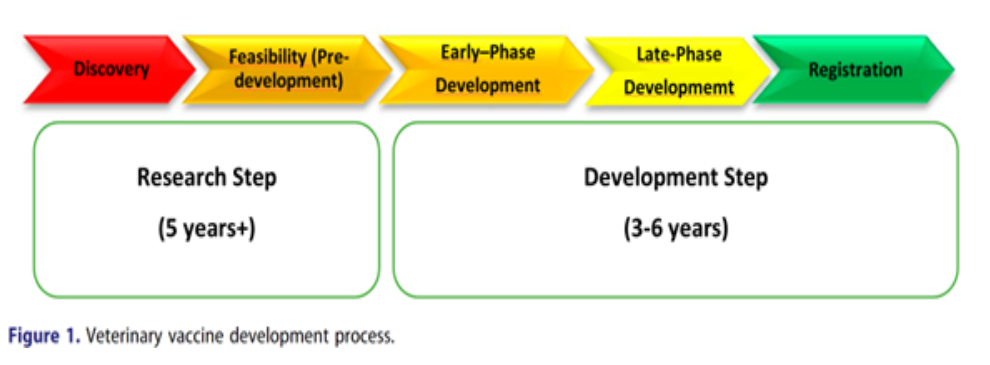
The veterinary vaccine development process is generally separated into a research step and a development step (Figure 1). The research step is further separated into a discovery or proof of concept phase and feasibility or pre-development phase. During discovery, exploratory studies are conducted on the biology of the target pathogen (virus, bacterium or parasite) and on the immunology of the target species (livestock or companion animal). This should lead to the development of a challenge model for the pathogen that can be used to facilitate the vaccine development process by enabling the protective immunity of a prototype vaccine to be assessed within the target species.
In addition, potential target antigens are identified for the vaccine, and experimental vaccine formulations are tested, leading to promising candidates for proof-of-concept efficacy studies, once a lead candidate vaccine has been identified, it can be taken into full commercial development.
Example of veterinary vaccine development:
At this early phase of development, if not before, some pre-master seed stocks of the candidate vaccine antigen or recombinant construct should be prepared. Alongside this, pre-seed stocks of any cell line used to produce the vaccine antigen are also prepared. Further characterization of the final vaccine can then be performed along with the development of quality control tests prior to the production of vaccine master seed banks, which will have been purified by cloning to ensure their consistency and homogeneity.
At this point, the project would move into the late phase of development and one or more of the consistency batches can be used for external good clinical practice (GCP) field trials to confirm the safety and efficacy of the vaccine within a wide range of animals of different age, sex and breed maintained under diverse husbandry conditions.
These field trials often provide the opportunity to compare the performance of a new vaccine to that of a licensed product in order to demonstrate its superiority or non-inferiority in terms of the immune response elicited.
In short, we can see in the picture below the macro product development of an animal health vaccine:
mRNA vaccines for veterinary use
mRNA vaccine technology is paving the way for new vaccines that are quicker and easier to modify and develop against emerging viruses. The COVID-19 pandemic confirmed how quickly mRNA vaccines can be designed and produced against a novel pathogen. Although only few mRNA vaccines have been specifically studied in protecting against animal infectious diseases in their natural hosts, the success of mRNA vaccines in humans has paved the way for advancement in veterinary medicine. Virus infections remain the major perceived threats to the global health and industrial livestock production.
The production process of mRNA vaccines can be broadly divided into six steps as follows.
(i) Sequence design: The sequence of the antigen is designed and optimized based on the sequence of the pathogen genome and inserted into the plasmid DNA.
(ii) In vitro transcription: Plasmid DNA is transcribed into mRNA by bacteriophage polymerases in vitro.
(iii) Purification: mRNA transcripts are purified by high performance liquid chromatography (HPLC) to remove contaminants and other reactants.
(iv) Nanoprecipitation: Purified mRNA is mixed with lipids in a microfluidic mixer to form lipid nanoparticles (LNP). Rapid mixing causes the lipids to encapsulate mRNA instantaneously and precipitate as self-assembled nanoparticles.
(v) Filtration: The nanoparticle solution is dialyzed or filtered to remove non-aqueous solvents and any unencapsulated mRNA.
(vi) mRNA vaccine: The filtered mRNA vaccine solution is stored in sterilized vials.
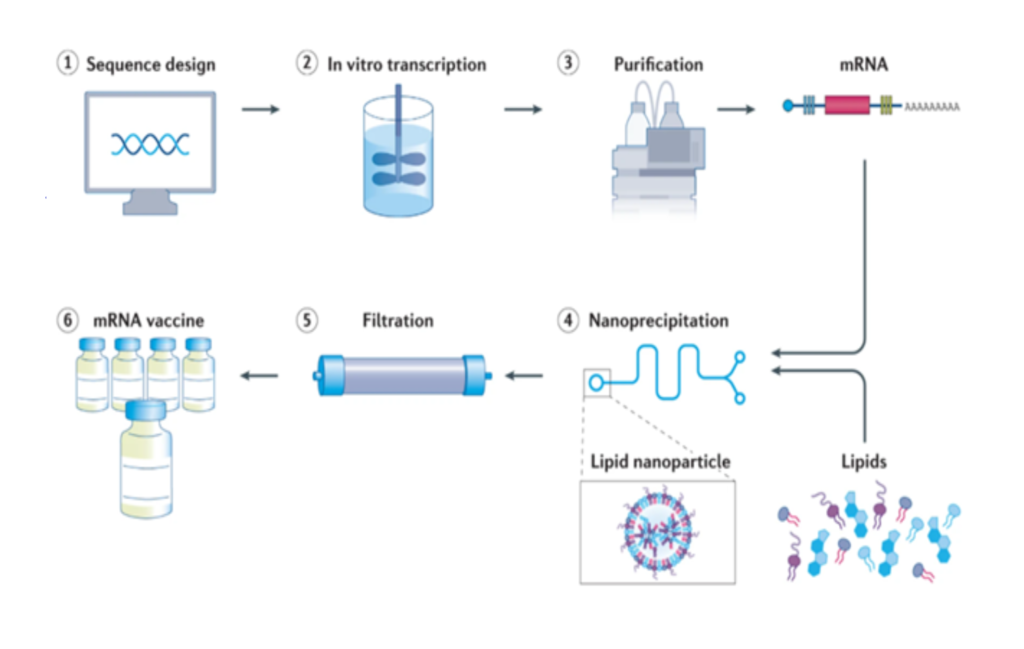
In a broad view this is the process to manufacture a mRNA vaccine. Please be noted that all mRNA vaccines, such as those developed by CureVac, use modified nucleotides and complex methods to stabilize such as nanoparticles and lipids, he explained. Despite this advanced technology, these vaccines still require storage at -70 Celsius or they would degrade to the point of non-functional, which is not a problem at all for veterinary use. The industry has been commercializing Marek vaccines in liquid nitrogen across the globe in extremely harsh environments without any logistic problems, hence this is a conquer challenge for veterinary vaccines.
Several targets are being studied for this technology, mainly because its production speed can be much faster than regular vaccines.
The potential candidates up to know are the following:
- Rift Valley Fever a potential zoonosis, transmitted to humans via contact with fluids and/or tissues from infected animals; vector borne transmission is also possible.
- Peste des Petits Ruminants
- East Coast Fever – tickborne disease that is caused by the apicomplexan organism, Theileria parva
- Bovine Respiratory Disease – is the most important cause of morbidity and mortality of intensively finished cattle. It has been estimated that BRD results in annual losses of USD 4.97 billion in the US alone
- Barber’s Pole Worm (Haemonchus contortus)
- mRNA Vaccines for Foot-and-Mouth Disease
- mRNA Vaccines for Rabies Disease
- mRNA Vaccines for Influenza
- mRNA Vaccines for Zoonotic Mosquito-Borne Flaviviruses
- T-Cell-Directed mRNA Vaccine for ASFV
- Polyvalent Mosaic mRNA Vaccine for PRRSV
- RBD-Based mRNA Vaccine for Animal Coronavirus
- E2-Based mRNA Vaccine for Pestiviruses
- Lumpy Skin Disease – lumpy skin disease virus
Some of the targets mentioned, like Rabies, FMD, and influenza, already have traditional vaccines that are effective in real-world conditions and are cost-effective. However, further research could lead to the development of vaccines that are more affordable and effective.
There are currently no mRNA vaccines licensed by the USDA/CVB but there are 2 products with similar technology that are being used (SEQUIVITY® IAV-S NA | Merck Animal Health USA (merck-animal-health-usa.com), The Sequivity product licensed and commercialized by MERCK is categorized by CVB as an RNA particle. In practical terms, this is derived from an alphavirus (Venezuelan equine encephalitis vaccine strain TC-83) virus that is defective. Like a MLV (Modify Live Virus) vaccine virus product, it can infect and delivery a viral payload, but the platform is defective and cannot create viable virus particles and so a non-replicating platform. The other product that will be produced is (Pandemic Preparedness or Panic Preservation? – Ceva), product was developed for the avian influenza outbreak in France for the AIH5 strain.
The AI virus possesses a segmented RNA genome, comprising eight segments of single-stranded RNA, with the virus proteins defined by two primary surface proteins, hemagglutinin and neuraminidase and the combination of HA and NA proteins determines the subtype of the virus. For example, H5N1 has HA type 5 and NA type 1. Some examples are H5N1, H7N9, H5N8 and H9N2.
The several outbreaks since 2015 that lead to the culling of millions of birds led the French government has placed orders for approximately 68 million vaccine doses from companies such as Ceva Santé Animale and Boehringer Ingelheim for the upcoming vaccination campaign. France aims to vaccinate about 60 million ducks over the next year as part of a broader strategy to combat bird flu and mitigate the economic impact on the poultry industry. Although specific figures for France alone are not readily available, the global avian influenza vaccine market is expected to reach USD 1.18 billion by 2032, with a projected annual growth rate of 7.5% from 2023 onward.
The mRNA technology has demonstrated successful utilization in combatting a highly impactful and deadly virus, SARS-CoV-2. Due to its production efficiency, a vaccine was developed and tested remarkably quickly, benefiting millions of people. However, considering the established efficacy of other technologies, such as FMD, Rabies, and clostridia, it becomes challenging to justify replacing them with mRNA technology. Moreover, the substantial investment required for such projects raises questions about their added value for animals.
In veterinary medicine, the standard procedure during virus or disease outbreaks involves the slaughter of animals in the “stamping out” process or the termination of susceptible animals within a 50 km radius. This method is regarded as safe and highly reliable.
As of now, the only veterinary medicine project that appears feasible is the CEVA product. The government’s preference for vaccination over ‘stamping out ‘animals, due to numerous outbreaks over several decades, has made the CEVA product a significant demand. However, it’s crucial that any other mRNA products undergo thorough validation and discussion within the veterinary medicine community, ensuring that all voices are heard in the decision-making process.
In conclusion, mRNA vaccines have potential in the veterinary field against animal infectious diseases, particularly the zoonoses. The advantages include no risk of infection or reversion to virulence, simultaneous immunizations against multiple pathogens, and relatively ease of design, enabling the RNA vaccine platform to overcome the bottlenecks faced by the traditional platform. Nonetheless, setbacks such as the instability of the vaccines, the relatively high expense of manufacturing them, and current delivery limitations impede the use of mRNA vaccines in the veterinary field. Currently, intramuscular, intradermal, or subcutaneous injection are the main delivery routes for mRNA vaccines in human. To reduce the stress, pain and cost of vaccinations, noninvasive delivery routes, including oral delivery and aerosol administrations, are becoming the preferred routes for animal vaccines. Thus, the design of new carriers should help improve the efficacy and stability of mRNA vaccines for the noninvasive delivery routes. However, the cost of mRNA vaccines remains high due to the use of high-priced reagents and the cold chain infrastructure. Given the fast pace of progress in mRNA vaccines, especially in the areas of immune-informatics and in vivo delivery methods, the future of mRNA vaccines at a low cost and high quality in the veterinary field is bright.
References:
Penn Vet | News Story detail (upenn.edu)
A Brief Overview of Emerging Vaccine Technologies for Pandemic Preparedness – PMC (nih.gov)


Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Thank you so very much for your comments! Please ask as many questions as you can!!
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://www.binance.com/en-IN/register?ref=UM6SMJM3
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? https://www.binance.com/bn/register?ref=UM6SMJM3
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Your article helped me a lot, is there any more related content? Thanks!