In order for any vaccine to be manufactured in GMP conditions it is necessary to first produce a ‘Master Seed Bank’ which is basically a bank of cells, bacteria, virus and or yeast with enough the vaccine manufacture for the product life cycle. The cell bank is the progenitor of all batches of the vaccine which are subsequently produced. In early phase development a batch for clinical use is typically produced directly from the master seed whereas for large scale manufacture a large working seed stock will be produced from the master, and batches are produced from the working seed. The master seed must be consistent (clonal, not containing variants), well characterized and produced using materials suitable for GMP manufacture, in a GMP certified clean room. All starting materials and raw materials should be fully traceable. A certificate of analysis is required before the master seed can be transferred to a second GMP manufacturer.
Frequently materials produced in research labs are not suitable for use as starting materials to generate a master seed due to lack of information about the history of the materials, or potential for contamination with other micro-organisms. The cleanest solution is frequently to re-derive the starting materials by e.g. transfection of a GMP-certified cell bank with plasmid DNA or viral DNA that has been phenol/chloroform extracted to remove all protein and therefore TSE (Transmissible spongiform encephalopathies risk), followed by cloning.
Novel mammalian cell banks will require testing for any possible adventitious agents and the risk of TSE contamination may be mitigated by extensive dilution during generation of the seed stock. Assays to characterize the product (identity, purity, potency) should be defined prior to producing the master seed. Assays for purity should test for any other vaccines/viruses/bacteria that were handled in the lab producing the starting materials as well as for mycoplasma if mammalian cells were used.
The principle also applies for virus, bacteria and fungus when necessary for the production of seeds for vaccines production. In case of recombinant proteins and molecules additional tests are required, however we will describe then as tumorgenicity, mutagenicity etc.
Critical Steps
Definition of the starting materials that will be used to generate the master seed.
- Are all components fully traceable? Is all documentation available?
- What animal-derived materials have been used? If bovine serum has been used was it from a TSE-free source? Bacterial growth medium frequently contains animal-derived components.
- A risk assessment should then be completed and used to define the production and testing strategy for the master seed
- Will cloning be required?
- Generation of the starting materials should then take place in isolation from other lab work to avoid any possible contamination with other materials
- Starting materials and required raw materials are transferred to a GMP clean room for master seed production and vialling
Testing of the master seed is conducted according to the agreed specification and a certificate of analysis is issued
Concepts:
- Master cell banks (MCBs) – an aliquot of a single pool of cells that generally has been prepared from the selected cell clone under defined conditions, dispensed into multiple containers, and stored under defined conditions. The MCB is used to derive all working cell banks (WCB
- Working cell banks (WCBs) – The WCB is prepared from aliquots of a homogeneous suspension of cells obtained from culturing the MCB under defined culture conditions
- Research cell banks (RCBs) – Research Cell Bank (RCB): This cell bank acts as a foundation for the development of a master cell bank. As the name suggests, it is used for research and development purpose
- End-of-production cell banks (EoPCBs) – An End of Production Cell Bank (EoPCB) is sometimes referred to as a Post-Production Cell Bank (PPCB) or as cells at the limit of in vitro cultivation. These cells are tested to validate the production system. This ensures that the cells are stable and that there are no contamination issues within the system.
- Cryoprotection Agents – agents used to protect the cells, virus, bacteria, parasites, fungus, etc during the freezing process.
Production procedure:
Once the virus, bacteria, fungus, or clone has been successfully identified and isolated and the specific cell line has been created, we can advance to the crucial stage of manufacturing the master cell bank. For those seeking further information, although the comprehensive procedures for virus, bacteria, or fungus isolation and cell lineage production will not be elaborated upon in this article, a relevant virus isolation procedure can be accessed via the following link: [6.3: Isolation, Culture, and Identification of Viruses – Biology LibreTexts].
Tips for producing Cell banks:
- Must be a dedicated cGMP licensed facility
- Be prepared to make enough cryovials at least >500 vials, to a cell count of 12e6 viable cell
- Try to closed and automated single-use process
- Rapid FILL-IT automated fills over 500 vials within 20 minutes
- Validated controlled-rate freezing this is important specially for pH sensitive products.
- Process Certified broth qualified for sterility assurance
- Qualified Person (QP) batch release – mandatory in EU.
- Flexibility of transfer to any CMO for product manufacture
Mandatory tests for the EU *according to EU GMP

- Tests for the presence of bioburden (bacteria and fungi) should be performed on individual containers (1% of the total number but not less than two containers) of the MCB and WCB
- Tests for the presence of mycoplasma should be performed on the MCB and WCB. Current procedures considered adequate include both the agar and broth media procedures as well as the indicator cell culture procedure.
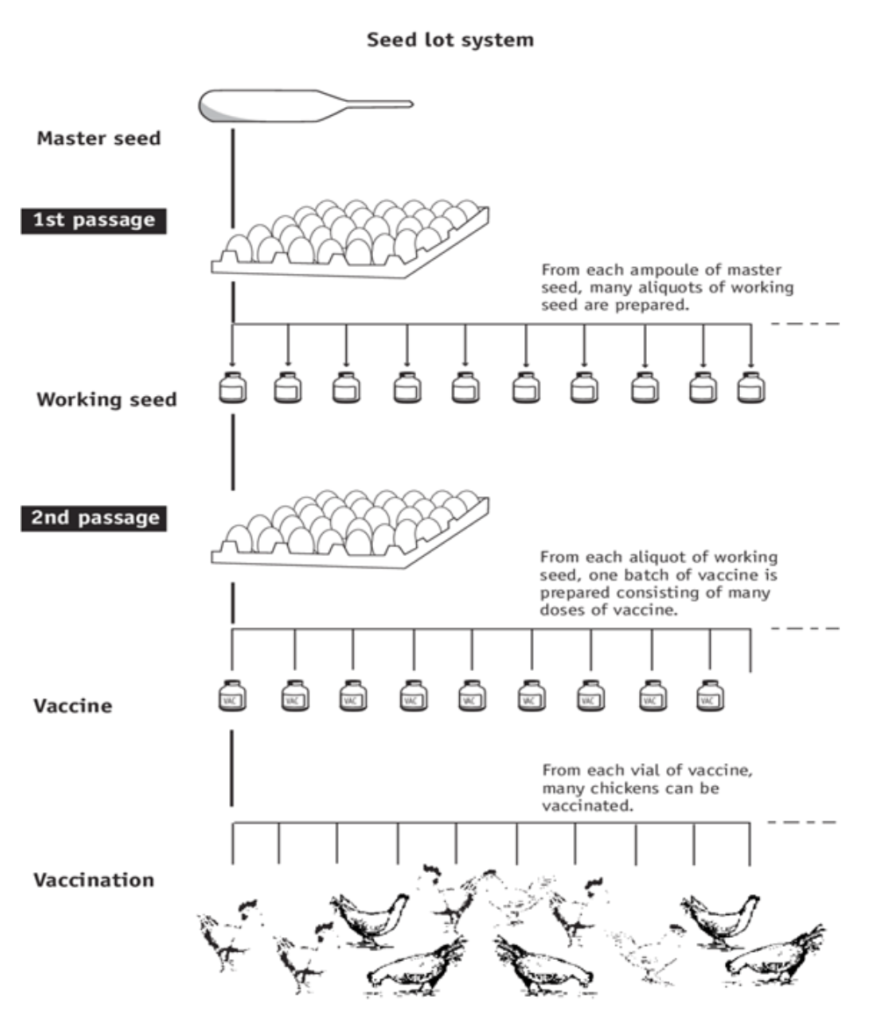
- When using eggs for virus propagation – Preparation of vaccines by a seed lot system reduces the chance of genetic alterations occurring during continuous passages in eggs. With each passage, there is the possibility of genetic alterations to the virus. These alterations may affect virulence, antigenicity and the yield of vaccine. A selected isolate is used to produce a volume of virus that comprises the master seed, which is divided into aliquots and stored. The master seed is usually freeze dried. An aliquot of master seed is used to produce a volume of working seed, which is again divided into aliquots and stored. An aliquot of the working seed is then used to produce a batch of vaccine. All batches of vaccine are then only two passages removed from the master seed.
- Cooling rate of 1°C per minute from ambient temperature is effective for a wide variety of cells and organisms.
- material frozen in plastic vials will take longer to thaw than that in glass ampoules, and sometimes gentle agitation of the vial during warming will accelerate the thawing process.
- Do not to vigorously agitate vials containing fragile cells such as protists and mammalian cells
- To minimize the risk of contamination during reconstitution, disinfect the external surface of the vial
Schematic of the cell/virus bank:

Cryoprotection Agents
Many compounds have been tried as cryoprotection agents, either alone or in combination, including sugars, solvents and even serum. Although there are no absolute rules in cryopreservation, glycerol and DMSO have been widely used and traditionally have been the most effective agents for preserving living cells and organisms. Other cryoprotectants can be used as well such as poly- ethylene glycol, propylene glycol, glycerine, polyvinylpyrolidone, sorbitol, dextran and trehalose.
Below you can find a table of some recommended concentrations is general:
| Cell Type | No of Cells | Cryoprotection Agent | Temperature |
|---|---|---|---|
| Bacteria | 107/ml | Glycerol (10%) | – 60 °C |
| Bacteriophage | 108 pfu/ml | Glyerol (10%) | – 80°C |
| Fungi Hyphae Spores | Not specified106/ml | Glycerol (10%)Glycerol (10%) | -150°C- 80°C |
| Yeast | 107/ml | Glycerol (10%) | -150°C |
| Protozoa | 105-107/ml | DMSOO(5-10%) or Glycerol (10-20% | – 150°C |
| Algae | 105-107/ml | Methanol (5-10%)or DMSO (5-10%) | -150°C |
| Plant Cells | (2-20% cells) | DMSO (5-10%)+Glycerol (5-10%) | -150°C |
| Animal Cells | 106-107/ml | DMSO (5-10%)Or Glycerol (5-10%) | – 150°C |
| Hybridomas | 107/ml | DMSO (5-10%)+Serum (20%) | – 150°C |
| Stem Cells | 105-106/ml | DMSO (5-10%)+Serum (20-90%) | – 105°C |
| Non _Cellular Material | |||
| Plant viruses | Not specified | None | -80°C |
| Animal viruses Cell free Infected Cells | 106/ml | NoneDMSO (7%)+ FCS (10%) | – 80°C- 150°C |
| Plasmids | 106/ml | Glycerol (10°C) | – 150°C |
| Phage Libraries | Not specified | Glycerol (10°C) | – 150°C |
| DNA | *For non-replicable materials the concentration does not affect the ability to freeze the material, only the specific application for its intended use | Non | – 80°C |
| RNA | Non | – 80°C | |
| Protein | Non | – 80°C | |
| Serum | Non | – 80°C | |
| Multicellular | |||
| Embryos | 20 | 1.2-propanediol, glycerol or ethylene glycol | – 150°C |
| Tissues | * | OCT | – 80°C |
| Blood | * | Glycerol | – 150°C |
Recommended cell concentration:
| Suspension cells: | 5 x 106 cells/ml |
| Adherent cells: | 1 x 106 cells/ml |
| Very small cells: | 1 x 107 cells/ml |
The schematic below is a seed lot system for an egg bases vaccine:
References:
- Mastering Cell Bank Production, https://www.biopharminternational.com/view/mastering-cell-bank-production, Published 2015
- WHO Reference Cell Banks (RCBs), https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/who-reference-cell-banks, Published
- Mastering Cell Bank Production, https://www.biopharminternational.com/view/mastering-cell-bank-production, Published 2015
- ICH Q5D Derivation and characterisation of cell substrates used for production of biotechnological/biological products – Scientific guideline | European Medicines Agency (EMA) (europa.eu)
- Clinical Guidances | FDA
- Q5B Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products | FDA
- Q5B Guideline.pdf (ich.org)
- Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks, Annex 3, TRS No 978 (who.int)
- trs_978_annex_3.pdf (who.int)
- Directive 2009/41/EC of the European Parliament and of the Council on the contained use of genetically modified micro-organisms. | FAOLEX
- LexUriServ.do (europa.eu)
- Directive 2009/41/EC of the European Parliament and of the Council on the contained use of genetically modified micro-organisms. | FAOLEX


Your article helped me a lot, is there any more related content? Thanks!
We can generated some more. Is there any topic in specific that you would like to see here?
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Your article helped me a lot, is there any more related content? Thanks!
Your article helped me a lot, is there any more related content? Thanks!