While AI has been a popular topic, its practical application in veterinary vaccine production is still in its early stages. The potential of AI for data analysis, predictions, and epitope predictions is vast, and we are just beginning to explore how these approaches can be applied to the production of current vaccines, particularly in a cost-effective manner for veterinary use. This exploration opens up exciting possibilities for the future.
Revising some concepts poultry vaccines are mainly produced in eggs, and eggs are usually the “villains” of any vaccine producer because they are the source of contamination.
Chicken-egg-based vaccine production remains the most effective manufacturing method available.
In short, within 9-10 days of embryonic age, chicken embryos are usually inoculated with viruses in the allantoic cavity. After some incubation time, which varies depending on the virus, the allantoid fluid is harvested. Sometimes, the AF is purified and inactivated to be used as an Active Ingredient in vaccine production, and other times, it is used as it is with a small purification process. Today’s egg-based vaccine production processes have evolved in yield, automation, capacity, quality control, and production speed.
The vaccines can be produced in Clean Eggs: these eggs are produced under conditions similar to parent flocks for producing day-old commercial layer chicks. The same quality of eggs is the standard product used to produce killed vaccines for veterinary use in Europe, whereas SPF-eggs are generally required for this purpose in the USA, as well as live vaccines. The quality specifications for these so-called “Clean Eggs” are laid down by the customers in cooperation with the producers. The main criteria are cleanliness and fertility.
For several reasons, clean Eggs are preferred over SPF-Eggs for producing human flu vaccines. Firstly, the seasonal requirement for many eggs and the higher production cost of SPF eggs make their use impractical. One fertile egg is typically necessary to produce a single vaccine dose for a specific flu virus. Since a flu shot usually contains a combination of flu viruses, making one shot requires up to 3 eggs. If SPF eggs were necessary, flu vaccination would be unaffordable for many, especially in low-income countries. As an illustration of poultry vaccine production, a single SPF-Egg is sufficient to produce 17,000 doses of the IB vaccine. (1 egg ~ 10 ml of AF).
Adjusting the vaccination program for the source flocks allows clean Eggs to be produced without antibodies for particular disease agents, meeting customers’ requirements. The market volume for clean vaccine eggs is estimated to be 530 million eggs per annum.
SPF-Eggs, which stands for Specified Pathogen Free Eggs, are produced in strictly isolated conditions to ensure the absence of all disease agents and antibodies against these agents, as stipulated in the European Pharmacopoeia. They are commonly used for live vaccines and, in certain regions, for killed vaccines for veterinary purposes. It is estimated that the global usage of SPF-Eggs totals 60 – 65 million. Only three major producers worldwide have the expertise to meet the stringent quality standards in this field. (VALO BioMedia, Charles River Laboratories | Boldly challenge scientific possibility (criver.com), About — Thai SPF Co Ltd., About Us – Ovagen)
However, as you can imagine, both eggs are not sterile and can be heavily contaminated. This contamination can enter the egg and lead to bulk contamination of the final vaccine (How to investigate a contaminated emulsion: From raw material to the final Emulsion—E&S Adjuvants (esadjuvants.com)).
Hence, today’s significant implication is whether AI can forecast vaccine bulk contamination. Accomplishing this could lower rejections and allow for real-time monitoring, thereby delivering added benefits.
The key challenge lies in determining the right data to input into the AI for running simulations and conducting data analysis. Below, you’ll find a simulation in which, in collaboration with the egg producers, we predicted the contamination rate of a specific batch with 40% accuracy.
During these instances, the product parameters included the time of egg receipt, quantity of eggs delivered, lab information, egg set day, egg flock age, product day, egg receipt day, and egg receipt month. With these parameters, we obtained the following results:
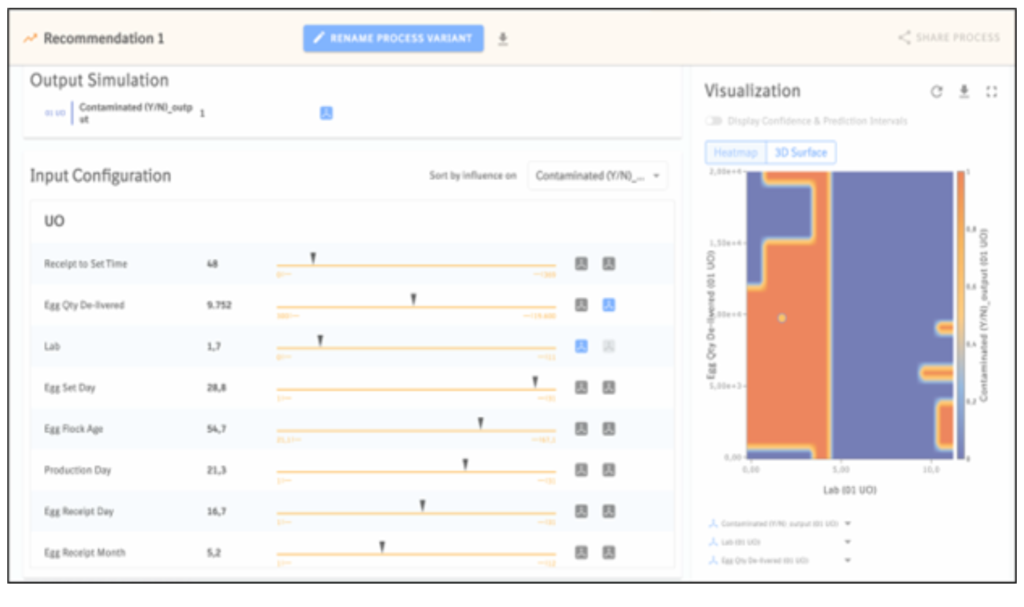
This “heat map” generated a risk score and a contamination rate as below:


After analyzing the results of this initial pilot case, we have obtained strong evidence, using artificial intelligence, indicating the potential contamination of the batch. As a result, the vaccine producers can now take proactive measures to prevent further contamination and mitigate potential damage.
AI candling process
One fascinating application of AI is in egg selection, where it is used to optimize the candling process. Candling involves using light to examine the eggs’ interior to determine the eggs’ quality and the status of the developing embryo. By harnessing AI technology, egg producers can automate and improve this process, leading to more efficient and accurate egg selection.
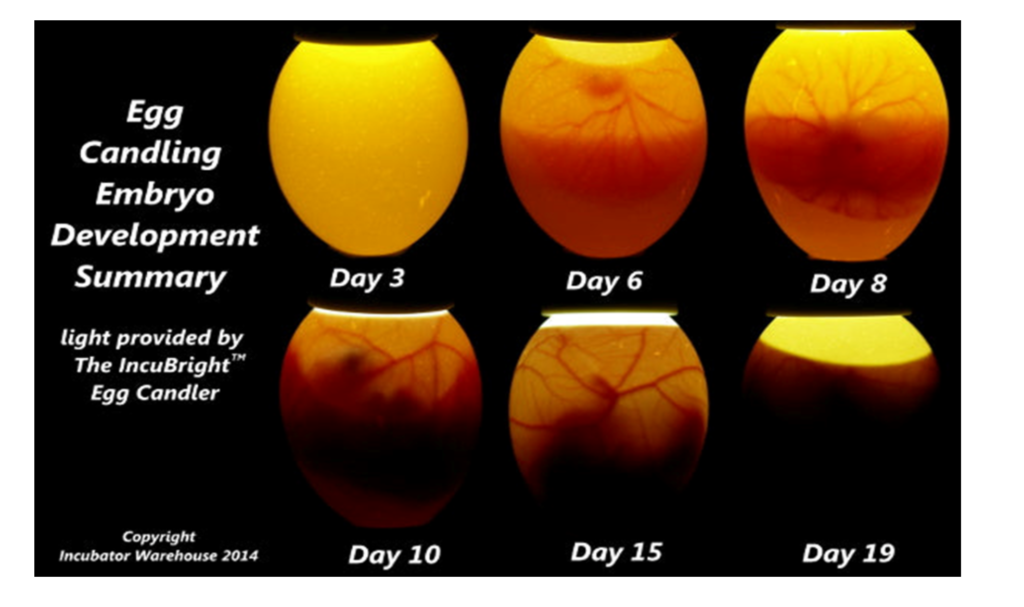
Candling is the process of using light to help determine the quality of an egg. Most egg producers use automated mass-scanning equipment to detect eggs with cracked shells and interior defects.
The equipment can “learn” how to candle the eggs using AI, reducing human errors, This setup is one of the many that we can find used in the system. (Orbem_AI-powered-Egg-Classification.pdf)
Challenges of Introducing AI in Bioprocessing
The pharmaceutical companies continue experiencing difficulties with delivering efficient end-to-end AI-based solutions in bioprocessing, including:
- Poor data quality – Bioprocessing environments are complex and generate high data volumes that need to be preprocessed and analyzed by data scientists to produce better outcomes.
- Low data quantity – Although biomanufacturing produces lots of data, it is common for the insights to be laid in data to be adequately collected or for the company to need more suitable infrastructure to handle large-scale datasets.
- Infrastructure challenges—Maintaining the biomanufacturing process is a challenge in itself. The industry requires new tools and strategies to support high-quality cell culture and upstream processing activities, including harvest operations, downstream purification steps, and formulation efforts.
- Lack of AI-skilled professionals – Only industry leaders have R&D departments filled with Data Scientists and ML experts who can provide the needed knowledge on-site. Other biopharmaceutical companies need to rely on partnerships for their projects.
- Lack of Regulatory response: The GMP regulatory environment is a slow-changing environment, meaning that any AI predictions or changes will take years to be approved and to change. Therefore, the lack of stimulation is also immense.
The biopharmaceutical industry can overcome challenges through digital transformation, which requires significant drug discovery and process development changes. Emerging technologies, such as AI-powered digital manufacturing, are being developed to meet these needs, enabling the capture of more data and the automation of complex tasks while offering real-time feedback and alerts on process performance without human intervention. Despite the promising future, it’s important to acknowledge that the pharmaceutical industry is known for its slow and bureaucratic nature, and implementing changes can often take years.


Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://accounts.binance.com/hu/register-person?ref=FIHEGIZ8
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. https://www.binance.com/register?ref=P9L9FQKY
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Your article helped me a lot, is there any more related content? Thanks!